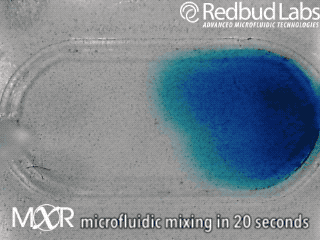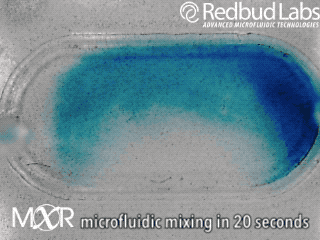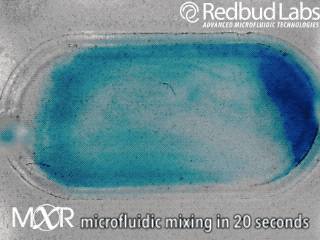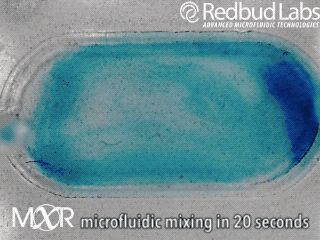
Why we invented MXR
Mixing is so obviously essential for life science assays that we rarely think about it until we discover there’s a problem with our assay. It takes too long, it doesn’t behave reliably, the sensitivity is poor. When we go to fix the problem, each of us solves the problem anew, with some workaround in the cartridge design.
Given how ubiquitous a problem microfluidic mixing is, the real question is why no one has offered a product like MXR before.
It’s not like there’s a lack of scientific work on the subject. But there are two big problems with the literature. For one thing, virtually all of the scientific work uses continuously pumped fluid instead of enclosed chambers. For another, cutting edge microfluidic technologies are virtually impossible to add to real-world consumable cartridges.
Mixing with zero dead volume
First: flow-through chambers. The standard mixing demo sets up a laminar flow condition using a Y-channel, then uses the mixing tech to disperse the laminar boundary. (Even we’ve done it.) A whole subcategory of mixing methods—the so-called “passive” methods—require this kind of fluid motion in order to work. This dramatically reduces their utility. While their instantaneous chamber volume may be small, but the total volume isn’t. This is not helpful for cartridge developers, because we have very strong opinions about the total sample volume. We also typically want our samples to stay put, because long channel runs take up space, and we want a small cartridge footprint. Also, during or after the mixing we’re probably going to measure something with a sensor in a fixed location. A traveling sample makes everything more complicated.
Our own work, published back in 2010 by Adam Shields and Briana Fiser et al., was a rare exception. We showed that biomimetic cilia could generate simultaneous pumping and mixing. The fluid they were pumping had to go somewhere, but because these micro-posts were in an enclosed chamber, the only place for it to go was in circles.
The punchline is that MXR makes any chamber a mixing chamber. No pumps. No plumbing. Zero dead volume.
Easy integration
Second: integration. There is no shortage of novel microfluidic devices in the world. Some are even manufacturable at workable scale and cost—for example, with a micro-machined injection molding tool, or commoditized semiconductor processing methods. But while those methods scale for small microfluidic chips, are they are rarely workable for the rest of the cartridge. In other cases, the novel method requires very precise flow rate control that is substantially constrains the design of the instrument. Novel methods often require additional electrical or mechanical contacts with the cartridge. And so on.
These nagging problems were the motivation behind MXR. It makes any chamber a mixing chamber, and it’s packaged and ready to be added right where you need it. For diagnostic cartridges, this easy integration makes all the difference in the world.
Our aim is to wrap our arms around ubiquitous microfluidic problems, and develop generic, powerful solutions that everyone in our industry can take advantage of. With MXR, our intentions is to solve microfluidic mixing, once and for all. We can’t wait to see what you do with it.
I hope you’ll try it out and let us know what you think.

400 Park Offices Dr.
Suite 301
RTP NC 27709
In The News

Redbud Labs exhibits at the 2025 APHL Annual Conference
Redbud Labs will exhibit at the APHL Annual Conference, held March 5–7, 2025, at the Oregon Convention Center in Portland, Oregon. The company will be located at Booth #415. The Redbud NA1 platform will be on display. NA1 is an automated, sealed-well system for...